E. J. Ko1,2 | J. Y. Hong1 | T.-R. Kwon1 | E. J. Choi1 | Y.-J. Jang1,3 | S. Y. Choi1,4|
K. H. Yoo5 | S. Y. Kim6 | B. J. Kim1
1Department of Dermatology, Chung-Ang
University College of Medicine, Seoul, South
Korea
2Department of Dermatology, Myongji
Hospital, Seonam University College of
Medicine, Goyang, Korea
3Department of Medicine, Graduate School,
Chung-Ang University, Seoul, Korea
4Department of Dermatology, Seoul Paik
Hospital, Inje University College of Medicine,
Seoul, Korea
5Department of Dermatology, College of
Medicine, Catholic Kwandong University,
International St. Mary’s Hospital, Incheon,
Korea
6Classys Inc., Seoul, Korea
Correspondence
Beom Joon Kim, Department of Dermatology,
Chung Ang University Hospital, 224-1
Heukseok-dong, Dongjak-ku, Seoul 156-755,
South Korea.
Email: beomjoon@unitel.co.kr
Abstract
Background: Noninvasive skin-tightening devices have become increasingly popular in response to increasing demand for improvements in skin laxity and tightening with minimal risk and recovery time.
Objective: We evaluated the efficacy and safety of HIFU for skin tightening in the face and body.
Methods: A total of 32 Korean subjects enrolled in this prospective clinical trial. The subjects were treated with HIFU to both cheeks, lower abdomen, and thigh. Skin elasticity was measured before and after treatment using a Cutometer (CT575, Courage and Khazaka®, Cologne, Germany). Three blinded, experienced dermatologists evaluated paired pre- and post-treatment (week 4 and 12) photographs according to the Global Aesthetic Improvement Scale (GAIS). Participants also completed selfassessments using GAIS. Subjects rated their pain on a numeric rating scale (NRS) immediately, 7 days, 4 weeks, and 12 weeks after treatment.
Results: Skin elasticity measured via a Cutometer was significantly improved 12 weeks after treatment at all treated sites (P<.05). Both IGAIS and SGAIS showed significant improvements 12 weeks after treatment. Immediately after treatment the mean NRS score was 3.00±1.586, but no pain was reported at 4 and 12 weeks post-treatment. No serious adverse effects were observed during the follow-up period.
Conclusion: HIFU safely and effectively improves skin elasticity and clinical contouring of the face and body.
KEYWORDS
body tightening, high-intensity focused ultrasound
1 | INTRODUCTION
The most common features of aging skin are laxity and loss of elasticity. As the skin ages, elastic fiber, collagen, and connective tissue in the dermis are reduced. Skin moisture and subcutaneous fat also decrease. There are many procedures to improve skin laxity, such as laser therapy, radiofrequency, botulinum toxin, fat autografts, and surgical lifting. Of these procedures, botulinum toxin and fat autografts are used for facial rejuvenation but are difficult to apply for improving body laxity. Radiofrequency and infrared laser devices which expose the dermis to controlled heat and stimulate neocollagenesis in dermis have inferior efficacy so that surgery still remains the treatment of choice in moderate to severe tissue laxity1.
Although surgical face lifting is the most effective treatment to improve skin laxity, it is also a procedure that involves risks such as scarring, infection, nerve damage, inherent risks of anesthesia, swelling, and bruising2.
HIFU technology was originally used as a non-invasive modality for selectively destroying tumor cells of internal organs by thermal coagulative necrosis for many decades3. HIFU was recently introduced as a new treatment modality for skin tightening and rejuvenation. The mechanism of HIFU is transcutaneous heat delivery to the deep dermis, subdermal connective tissue, and fibromuscular layer in precise microcoagulation zones at consistent programmed depths
without damage to the epidermis. This microcoagulation is thought to cause gradual tightening of the skin through collagen contraction and
remodeling4. HIFU first received approval for eyebrow lifting, but dermatologists are using the technology for many off-label applications,
such as facial rejuvenation, skin whitening, and lipolysis.
HIFU has been used safely and effectively to treat facial and neck skin in a variety of skin types, but some studies have examined its use
for the body, including our pilot study5-7. In this study, we sought to determine the clinical efficacy and safety of HIFU with novel transducers in both face and body regions.
2 | SUBJECTS AND METHODS
Korean patients with skin laxity on the face, abdomen, and thigh were recruited for study entry. The study was approved by the Institutional Review Board of Chung-Ang University Hospital. Informed consent was obtained from all patients. Exclusion criteria were prior cosmetic or surgical treatments (eg, laser, RF, surgical lifting, filler injections), skin infection or inflammation, pregnancy, skin diseases that may alter wound healing, open wounds, and scarring over the treatment area.
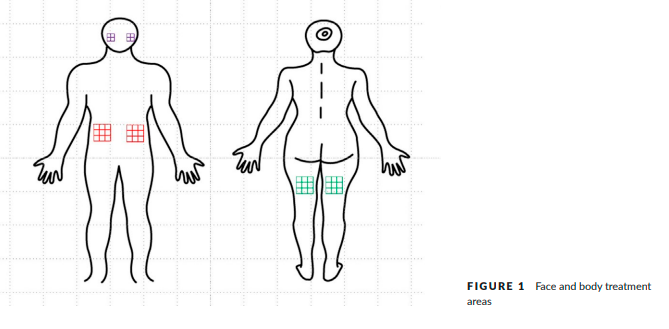
For pre-treatment preparation, we applied topical anesthetic cream to all treated areas including both cheeks, the lower abdomen, and the posterior thigh. The sizes of the involved areas were 5.0 × 5.0 cm2 on each cheek and 7.5 × 7.5 cm2 on each lower abdomen and thigh
(Figure 1). We used a HIFU device (ULTRAFORMER III (SHURINK) CLASSYS INC., Seoul, Korea) with five different transducers: one basic transducer for facial skin tightening (MF1: 7-MHz, 1.5-mm focal depth), and four newly developed transducers for body skin tightening (MF3: 2-MHz, 3.0-mm focal depth, MF4: 2-MHz, 4.5-mm focal depth, MF6: 2-MHz, 6.0-mm focal depth and MF9: 2-MHz, 9.0-mm focal depth).
Ultrasound gel was applied to the treated area and the transducer of HIFU was pressed perpendicularly, uniformly, and firmly to the skin surface (Figure 2). Treatment exposure was initiated with a line of individual ultrasound pulses. The pulse duration for each individual exposure ranged from 25 to 40 milliseconds. The 25-mm-long exposure lines of ultrasound pulses were manually delivered adjacent and parallel to one another approximately 3–5 mm apart. We treated subjects with several types of transducers appropriate to the thicknesses of facial and body skin. Three transducers (MF1, 3, and 4) were applied to the face and all five transducers (MF1, 3, 4, 6, and 9) were applied to the body. The energy per ultrasound pulse ranged from 1.0 to 1.5 J. When patients reported feeling pain, we reduced exposures to 0.1–0.3 J per time, and did not increase exposures up to 1.5 J.
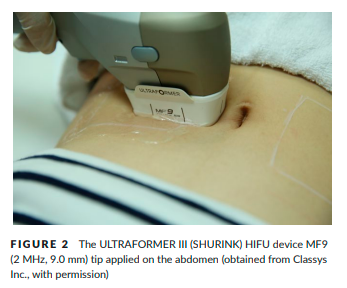
The treatment lines included a total of 120 shots for the cheek, distributing a total 537.6 J, and 450 shots for the abdomen and thigh, distributing a total 900 J. The time required for complete HIFU treatment of the face and body was over 40 minutes.
All patients were followed up at 4 and 12 weeks after treatment, at which times we obtained clinical photographs using consistent patient positioning, camera settings (Canon EOS 600D, high-resolution setting, 5760 × 3840 pixels, Canon Inc., Tokyo, Japan), and room lighting. Baseline and post-treatment photographs were randomly displayed, and independently evaluated by three dermatologists who were masked to the study protocol. Investigator Global Aesthetic Improvement Scale (IGAIS) scores were determined using side-byside comparisons of 4- and 12-week post-treatment photographs to baseline. The subjects also evaluated the tightening effects using the Subject Global Aesthetic Improvement Scale (SGAIS) at 4 and 12 weeks post-treatment. We used the Cutometer (Courage+Khazaka Electronic GmbH, Cologne, Germany) to measure skin elasticity and objectively evaluate skin tightening. Among the cutometer-specific R values (R0–R9), the R7 value is the ratio of elastic recovery to the total deformation and represents biological elasticity. Adverse effects were assessed at each visit after treatment. A numeric rating scale (NRS) was used to score pain immediately, 7 days, 4 weeks, and 12 weeks after the application of HIFU.
2.1 | Statistical analyses
Statistical analyses were performed using SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL, USA) and R version 3.2.3 (2015-12- 10). We used Hochberg step-up methods to adjust values for multiple comparisons. Statistical comparisons before and after treatments were performed using paired t tests. Data are presented as mean±standard deviation. P values <.05 were considered statistically significant.
3 | RESULTS
3.1 | Efficacy
This study included 32 Korean patients (29 females and 3 males), aged 21–59 (mean±SD: 44.47±9.73) with Fitzpatrick skin types III and IV. All patients completed the 3-month study.
The mean R7 value according to the Cutometer was significantly increased at 4 and 12 weeks post-treatment compared to baseline in all treated areas (Figure 3).
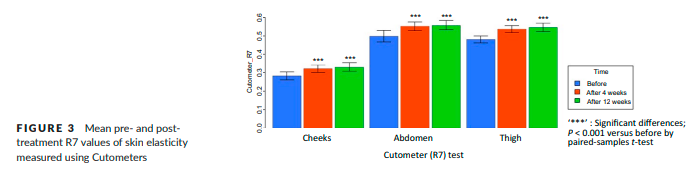
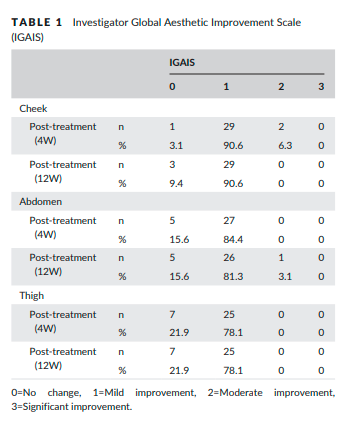
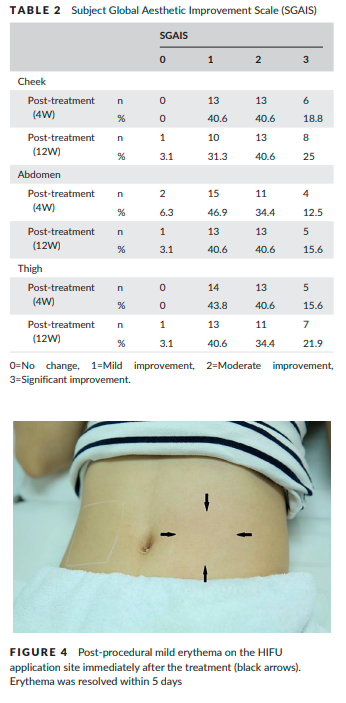
The change of the mean R7 value at the thigh was 0.054±0.032, which represented the greatest change among the treated areas. IGAIS scores also showed good results (Table 1). Of the three treated areas, the cheek demonstrated the greatest improvements after treatment. At 4 weeks post-treatment, the improvement rates of subjects who were assessed as either improved (IGAIS score 1) or much improved (IGAIS score 2) were 96.9%, 84.4%, and 78.1% on the cheek, abdomen, and thigh respectively. At 12 weeks post-treatment, the improvement rate of the cheek area was reduced to 90.6%, but the body areas did not change significantly. Most subjects were satisfied with the results of treatment (Table 2). At 4 weeks post-treatment, all subjects rated SGAIS scores as greater than 1 on the cheek and thigh. The improvement rate assessed for the abdomen as greater than SGAIS 1 was 93.8%. At 12 weeks post-treatment, the improvement rates of cheek and thigh were reduced from 100% to 96.9%. However, the improvement rate of the abdomen increased to 96.8%.
3.2 | Safety
The mean pain scores immediately and at 7 days after treatment were 3.00±1.586 and 0.031±0.177, respectively. The degree of pain
decreased substantially within the first week post treatment. All patients were able to complete the treatment. No subjects experienced
persistent pain over the treatment areas at 3 months follow-up.
Erythema was seen in up to 9.38% of the treatment sessions immediately post-treatment, but mostly subsided within 5 days (Figure 4).
No patients showed surface injury or thermal damage on the treatment site. Ecchymosis was seen in up to 6.25% of treatment sessions
immediately post-treatment. By 3 days post-treatment, all cases of ecchymosis had resolved. We observed no serious or delayed adverse effects during the follow-up period.
4 | DISCUSSION
There are many noninvasive options of body sculpting, such as radiofrequency ablation, cryolipolysis, injection lipolysis, external low-level lasers, laser ablation, nonthermal ultrasound, and HIFU. Each of these treatments has no admission for treatment without anesthesia or analgesia and typically fewer complications than liposuction. However, with the exception of HIFU, patients have to visit the hospital several times for multiple treatments to achieve meaningful. Injection lipolysis and cryolipolysis have significant potential for AEs, which is largely unregulated and may cause significant pain, hematoma, allergic reactions, necrosis, scarring, panniculitis, and rapid release of lipids into the bloodstream. In contrast, previous clinical studies supported thermal HIFU for body sculpting have had no serious AEs including alterations in lipid profiles or other laboratory parameters5-8.Therefore, many clinician are keeping an eye on the HIFU technique as purpose of body sculpting.
Studies of HIFU facilitate the understanding of mechanisms of action for body sculpting. When used for body sculpting, HIFU delivers focused, high intensity ultrasonic energy to deep subcutaneous tissue, producing heat capable of effectively ablating adipocytes and thermally modifying collagen within the tissue matrix. In addition to local adipocyte necrosis, evidence of collagen remodeling from the thermal effects of HIFU has been observed9. Application of HIFU at a frequency of 1 MHz to adipose tissue leaves collagen fibers intact, but at frequencies of 2–3 MHz, diffuse contraction of collagen fibers occurs. Histological analyses performed after the procedure confirm
that HIFU disrupts or denatures collagen fibers, resulting in new collagen formation accompanied by a general tightening of the septal fibers and skin9.
Based on these results, newly developed transducers for application to body sites at a variety of focal depths (3.0–9.0 mm) are deemed to be suitable for body tightening.
Also, we found no thermal damage on the skin surface of the HIFU treatment site. Kwon et al. has reported the temperature changes of
the porcine model during HIFU procedure, which showed targeted subcutaneous fat to be around 70°C, while the skin surface temperature only went up to 33.1–35.6°C10. Therefore, we hypothesized that newly developed transducers could effectively and safely deliver HIFU energy deeper into the skin and eventually show body sculpting effects due not only to skin tightening but also to the reduction of subcutaneous fats.
In this study, we used the Cutometer to evaluate the skin tightening effects of HIFU. Objective measurements of skin elasticity after laser, radiofrequency, and HIFU treatments are desirable. The use of uniform photographic documentation has improved, but there are often still inconsistencies in patient position and lighting. Physicianbased grading systems are characterized by inherent elements of subjectivity. The purely objective quantification of results would be of great benefit for the evaluation of skin tightening procedures.
There are several reports describing the quantification of facial rejuvenation results using Cutometers. These include Shin et al., who used
Cutometers to assess the effectiveness of photographic rejuvenation with intense pulsed light (IPL)11. Similarly, Naouri et al. assessed improvements in skin tightness after applying CO2 fractional lasers12. Ahn et al. demonstrated a stronger relationship between aging and skin elasticity parameters (R2, R7) than between aging and skin viscoelasticity parameters using Cutometers (R6),13 while Kruger et al.
made similar observations by conducting cutometric tests in a group of 120 females treating various parts of the body (cheek, neck, neckline, forearm, and back of the hand). They recommended the application of parameters R2 and R7 to evaluate the process of skin aging14.
Thus, this study determined the R7 value from nine parameters of Cutometer.
In this study, we observed significant improvements in two body regions (abdomen and thighs) as well as the cheek when targeted for HIFU treatment. Adverse effects were limited to transient pain in most patients and occasional erythema or ecchymosis in some patients. HIFU can be safely and effectively used to improve the clinical appearance of the abdomen and thighs. Therefore, HIFU could meet current demands for significant, noninvasive skin lifting
and tightening. Tightening and lifting of facial and body skin laxity can be achieved by inducing collagen fiber contraction and stimulating de novo collagenesis. By using newly developed transducers with different energy outputs and focal depths, HIFU treatment can be tailored to meet the unique physical characteristics of each patient.
CONFLICTS OF INTEREST
Not declared.
REFERENCES
1. MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18-25.
2. Northington M. Patient selection for skin-tightening procedures. J Cosmet Dermatol. 2014;13(3):208-211.
3. Laubach HJ, Makin IR, Barthe PG, Slayton MH, Manstein D. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34(5):727-734.
4. Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32(1):71-77.
5. Choi SY, No YA, Kim SY, Kim BJ, Kim MN. Tightening effects of highintensity focused ultrasound on body skin and subdermal tissue: a pilot study. J Eur Acad Dermatol Venereol. 2016;30(9):1599-1602.
6. Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg.
2012;38(5):754-759.
7. Solish N, Lin X, Axford-Gatley RA, Strangman NM, Kane M. A randomized, single-blind, postmarketing study of multiple energy levels of high-intensity focused ultrasound for noninvasive body sculpting. Dermatol Surg. 2012;38(1):58-67.
8. Shek SY, Yeung CK, Chan JC, Chan HH. Efficacy of high-intensity focused ultrasonography for noninvasive body sculpting in Chinese
patients. Lasers Surg Med. 2014;46(4):263-269.
9. Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28:257-262.
10. Kwon TR, Im S, Jang YJ, et al. Improved methods for evaluating pre-clinical and histological effects of subcutaneous fat reduction
using high-intensity focused ultrasound in a porcine model. Skin Res Technol. 2017;23(2):194-201.
11. Naouri M, Atlan M, Perrodeau E, et al. Skin tightening induced by fractional CO2 laser treatment: quantified assessment of variations in mechanical properties of the skin. J Cosmet Dermatol. 2012;11:201-206.
12. Hedelund L, Due E, Bjerring P, Wulf HC, Haedersdal M. Skin rejuvenation using intense pulsed light: a randomized controlled split face trial with blinded response evaluation. Arch Dermatol. 2006;142(8):985-990.
13. Ahn S, Kim S, Lee H, Moon S, Chang I. Correlation between a Cutometer® and quantitative evaluation using Moire topography in age-related skin elasticity. Skin Res Technol. 2007;13(3):280-284.
14. Krueger N, Luebberding S, Oltmer M, Streker M, Kerscher M. Agerelated changes in skin mechanical properties: a quantitative evaluation of 120 female subjects. Skin Res Technol. 2011;17(2):141-148.
How to cite this article: Ko EJ, Hong JY, Kwon T-R, et al.
Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res Technol.
2017;00:1–5. https://doi.org/10.1111/srt.12371
